Jan 30, 2023Dipole Moment. When two electrical charges, of opposite sign and equal magnitude, are separated by a distance, an electric dipole is established. The size of a dipole is measured by its dipole moment (\(\mu\)). Dip ole moment is measured in Debye units, which is equal to the distance between the charges multiplied by the charge (1 Debye eq uals \(3.34 \times 10^-30\; C\, m\)).
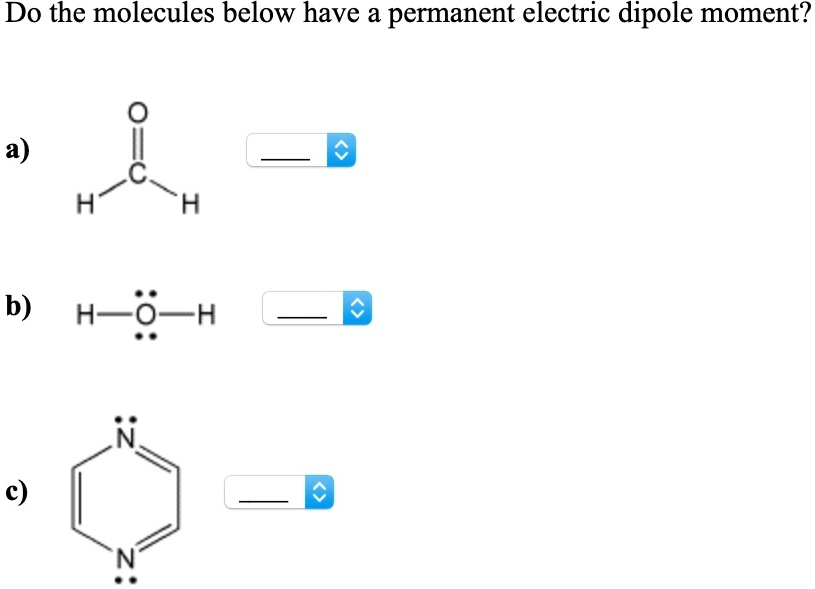
Solved Do the molecules below have a permanent electric | Chegg.com
Molecular Dipole Moments. In molecules containing more than one polar bond, the molecular dipole moment is just the vector combination of what can be regarded as individual “bond dipole moments”.Mathematically, dipole moments are vectors; they possess both a magnitude and a direction.The dipole moment of a molecule is therefore the vector sum of the dipole moments of the individual bonds in
Source Image: numerade.com
Download Image
The magnitude of a bond dipole moment is represented by the Greek letter mu ( µ) and is given by the formula shown below, where Q is the magnitude of the partial charges (determined by the electronegativity difference) and r is the distance between the charges: μ = Qr μ = Qr
Source Image: numerade.com
Download Image
A Homonuclear Molecule with a Permanent Electric Dipole Moment | Science The typical argument on a possible permanent electric dipole moment in molecules and the resulting rotational and vibrational spectra goes as follows [4]: In the case of atoms, the permanent electric dipole moment is always zero for non-degenerate states because they are eigenstates of the parity operator and D is odd under this transformation.

Source Image: sciencedirect.com
Download Image
Do The Molecules Below Have A Permanent Electric Dipole Moment
The typical argument on a possible permanent electric dipole moment in molecules and the resulting rotational and vibrational spectra goes as follows [4]: In the case of atoms, the permanent electric dipole moment is always zero for non-degenerate states because they are eigenstates of the parity operator and D is odd under this transformation. In that case, the imposition of an external electric field will exert a torque on the molecules, and will cause all their dipole moments to line up in the same direction, and thus the bulk material will acquire a dipole moment. The water molecule, for example, has a permanent dipole moment, and these dipoles will align in an external field.
Permanent Electric Dipole Moments of Atoms and Molecules – ScienceDirect
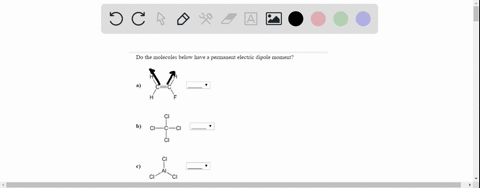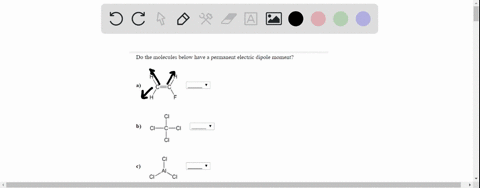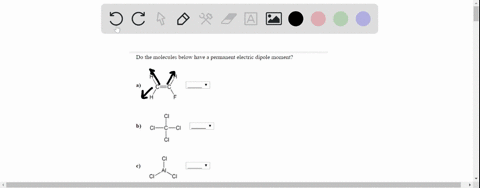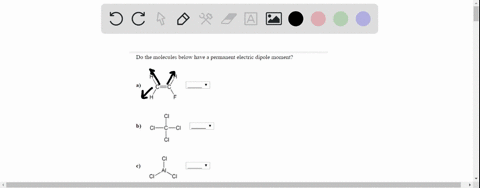
On the “permanent electric dipole moment” in molecules Guglielmo M. Tino Dipartimento di Fisica e Astronomia and LENS Laboratory Università di Firenze and INFN-Sezione di Firenze via Sansone 1, Sesto Fiorentino, Italy email: [email protected] December 13, 2021 Abstract Solved Do the molecules below have a permanent electric | Chegg.com

Source Image: chegg.com
Download Image
Atkins & de Paula: Atkins’ Physical Chemistry 9e – ppt video online download On the “permanent electric dipole moment” in molecules Guglielmo M. Tino Dipartimento di Fisica e Astronomia and LENS Laboratory Università di Firenze and INFN-Sezione di Firenze via Sansone 1, Sesto Fiorentino, Italy email: [email protected] December 13, 2021 Abstract
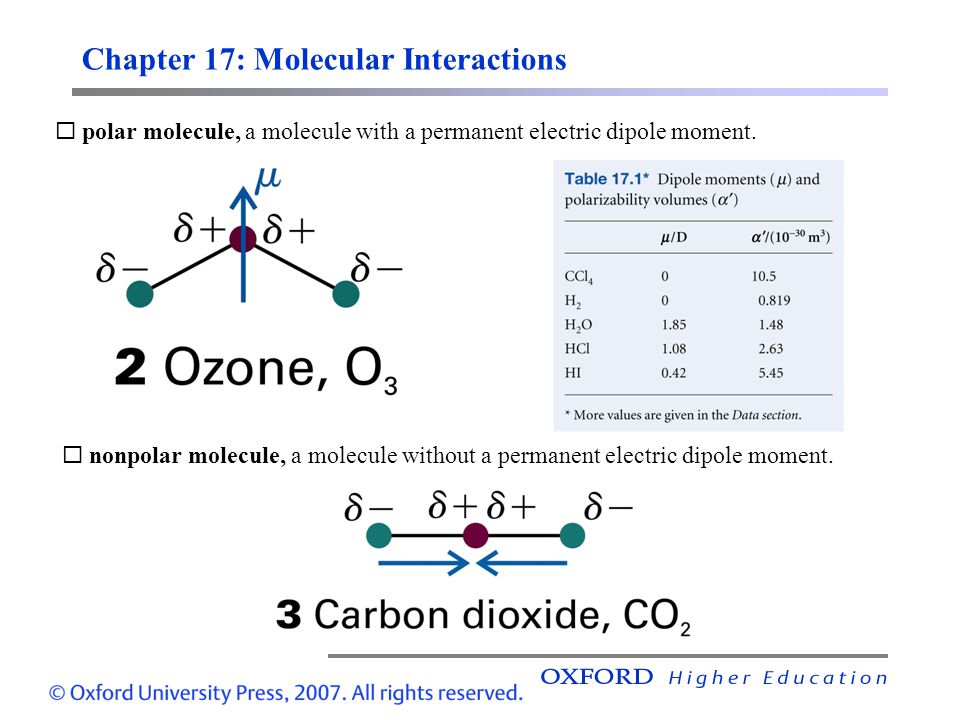
Source Image: slideplayer.com
Download Image
Solved Do the molecules below have a permanent electric | Chegg.com Jan 30, 2023Dipole Moment. When two electrical charges, of opposite sign and equal magnitude, are separated by a distance, an electric dipole is established. The size of a dipole is measured by its dipole moment (\(\mu\)). Dip ole moment is measured in Debye units, which is equal to the distance between the charges multiplied by the charge (1 Debye eq uals \(3.34 \times 10^-30\; C\, m\)).
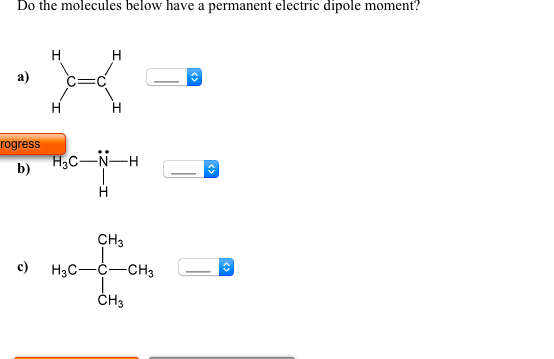
Source Image: chegg.com
Download Image
A Homonuclear Molecule with a Permanent Electric Dipole Moment | Science The magnitude of a bond dipole moment is represented by the Greek letter mu ( µ) and is given by the formula shown below, where Q is the magnitude of the partial charges (determined by the electronegativity difference) and r is the distance between the charges: μ = Qr μ = Qr

Source Image: science.org
Download Image
Why does SF4 have a dipole moment? – Quora Figure 1 To locate the molecule so specifically, we would need to give the x, y, and z coordinates of each of its atoms, i.e., (xA, yA, zA) = rA (xB, yB, zB) = rB which is a total of 6 numbers. However, we note that the molecule looks the same, no matter where in space it is located.
Source Image: quora.com
Download Image
Gen Chem 2 Q2 Module 2 | PDF | Chemical Polarity | Intermolecular Force The typical argument on a possible permanent electric dipole moment in molecules and the resulting rotational and vibrational spectra goes as follows [4]: In the case of atoms, the permanent electric dipole moment is always zero for non-degenerate states because they are eigenstates of the parity operator and D is odd under this transformation.

Source Image: scribd.com
Download Image
Asking the Right Question In that case, the imposition of an external electric field will exert a torque on the molecules, and will cause all their dipole moments to line up in the same direction, and thus the bulk material will acquire a dipole moment. The water molecule, for example, has a permanent dipole moment, and these dipoles will align in an external field.
Source Image: philippinesbasiceducation.us
Download Image
Atkins & de Paula: Atkins’ Physical Chemistry 9e – ppt video online download
Asking the Right Question Molecular Dipole Moments. In molecules containing more than one polar bond, the molecular dipole moment is just the vector combination of what can be regarded as individual “bond dipole moments”.Mathematically, dipole moments are vectors; they possess both a magnitude and a direction.The dipole moment of a molecule is therefore the vector sum of the dipole moments of the individual bonds in
A Homonuclear Molecule with a Permanent Electric Dipole Moment | Science Gen Chem 2 Q2 Module 2 | PDF | Chemical Polarity | Intermolecular Force Figure 1 To locate the molecule so specifically, we would need to give the x, y, and z coordinates of each of its atoms, i.e., (xA, yA, zA) = rA (xB, yB, zB) = rB which is a total of 6 numbers. However, we note that the molecule looks the same, no matter where in space it is located.